Understanding Extractable and Leachable Studies

Extractables and Leachable studies focus on how pharma products interact with their packaging. Product and packaging interaction can span from the production process to shelf life. While packaging is essential for the safe storage and/or administration of products, some packaging can negatively affect the contained product due to the migration or transfer of certain substances. To prevent excessive levels of migration and to protect consumers, packaging must meet certain standards and regulations.
Why Study Extractables & Leachables?
To determine migration levels, products must undergo extractable and leachable studies. Extractable compounds are those that can be drawn out of the regulated product. The contaminants are exposed when certain solvents are applied under specific conditions. On the other hand, Leachables are compounds that could migrate from any product’s drug formulation. Product contamination poses a toxicological risk, while also accelerating product degradation, especially for biopharmaceuticals.
Pharmaceutical packaging, bioprocess materials, drug delivery systems, or implantable medical devices construction is very complex. These products can contain a mixture of plastic, polymer, rubber, glass, printed surfaces and coatings. As such, extractable and leachable studies must be tailored to the specific product for accurate assessment.
Extractables & Leachable Study Process
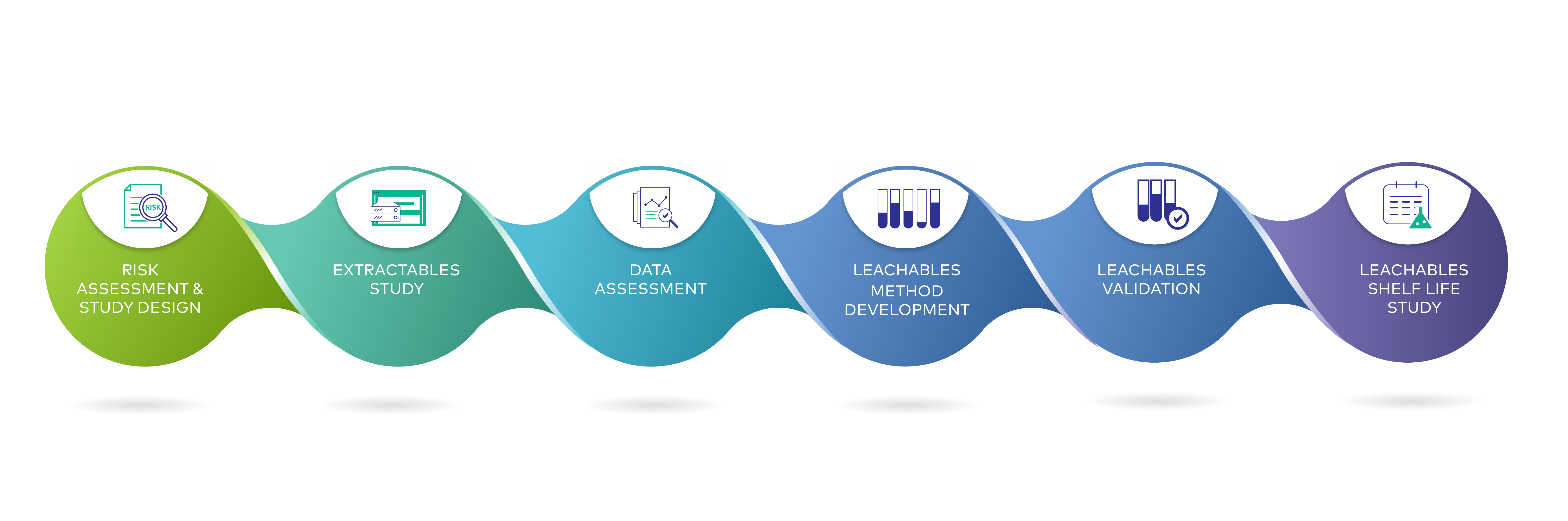
E&L studies are designed based on USP (US Pharmacopeia) (665/1665 or 1663/1664), ISO 10993, BPOG, ICH (International Harmonisation) and PQRI (Product Quality Research Institute) guidelines.
Guidelines to be used are selected based on the product to be analysed taking into consideration its chemical formulation, administration route, dosage and credibility with regulatory authorities. The selected study must scientifically justify the approach used for sample handling, analysis and reporting to help ensure regulatory compliance of the test product.
Extraction and Analytical Techniques
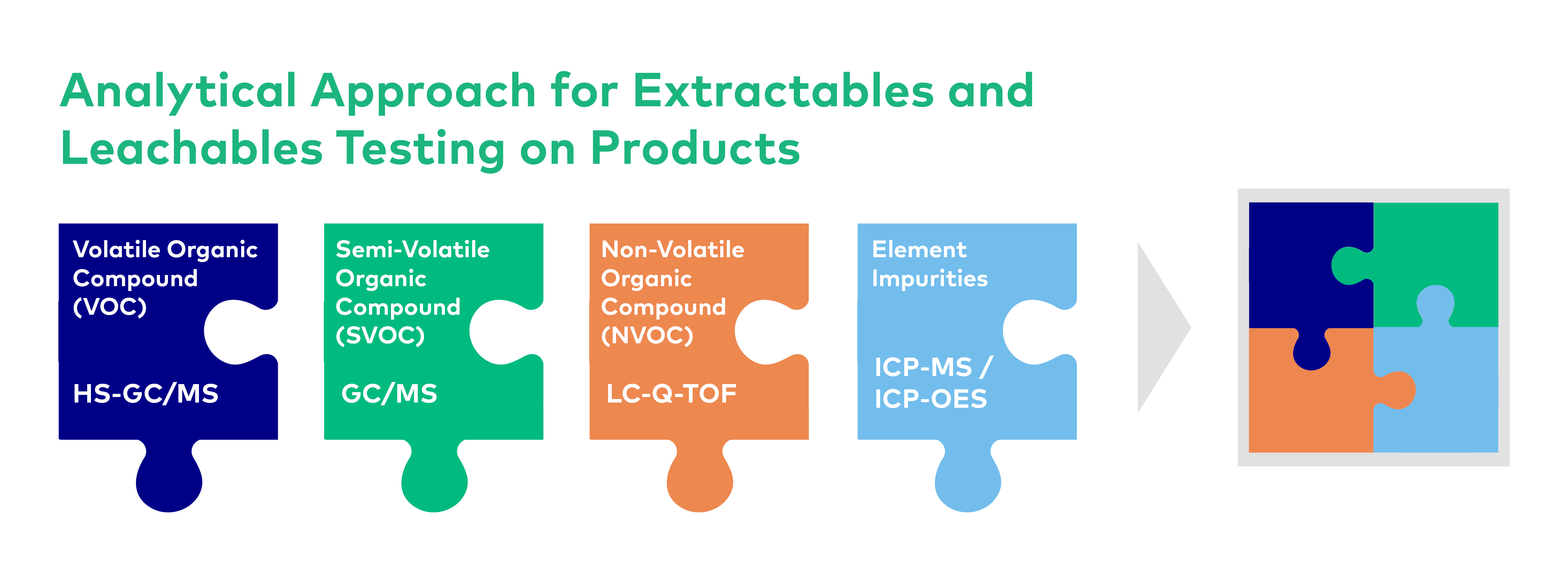
There is no single analytical technique that covers all the compound categories for E & L studies. Different study techniques are applied depending on the product and packaging components. As a result, testing is achieved by using complementary analytical techniques. To achieve this, we conduct an initial study to determine all possible sources of contamination. Potential extractable and leachable sources include antioxidants, plasticizers, dyes and metal catalysts, and polymer and degradation products.
Extraction techniques that can be used include Reflux, Soxhlet, Sonication, Microwave and Incubation at controlled temperature conditions (with agitation or recirculation if needed).
Similarly, different analytical techniques can be applied, such as:
- Qualitative screening by GC-MS and/or LC-Q-TOF and eluted compounds identification using NIST databases.
- Quantitative testing for:
- Volatile and semi volatile impurities: GC/MS, TDS-GC MS/MS, GC-FID
- Non-volatile impurities: UPLC-UV/MS/MS, HPLC-UV/PDA-FLD, LC-Q-TOF
- Anionic impurities: Ion chromatography
- Elemental impurities: ICP-MS, ICP-OES
Regulated Chemicals & Compounds
Regulated chemicals are required to meet migration limits set by regulatory bodies like USFDA, ANVISA, Health Canada and EMEA. Extractable and Leachable studies cover the following regulated compound classes:
|
|
Cotecna Pharma offers a wide range of services to support pharmaceutical clients with Extractable and Leachable studies. Through our 3 accredited Pharma laboratories Neotron in Europe and Shiva Laboratories and Geo-Chem based in India, we offer studies for the following products:
|
|
Our services tailored to client needs and products. Analysis is delivered by our expert teams using the best technology and techniques. To learn more about our extractable and leachable service or to request a quote, please contact us.
Latest News

24.07.2025
Geo-Chem Launches New Food Testing Lab in Unjha, Gujarat, India
Geo-Chem India expanded its scope of work in Gujarat with the launch of a new food testing laboratory.

08.04.2025
Innovation and Precision: How Complex Diagnostics Are Transforming Plant Health in the Field
Harnessing Technology for Smarter, More Sustainable Crop Protection.

